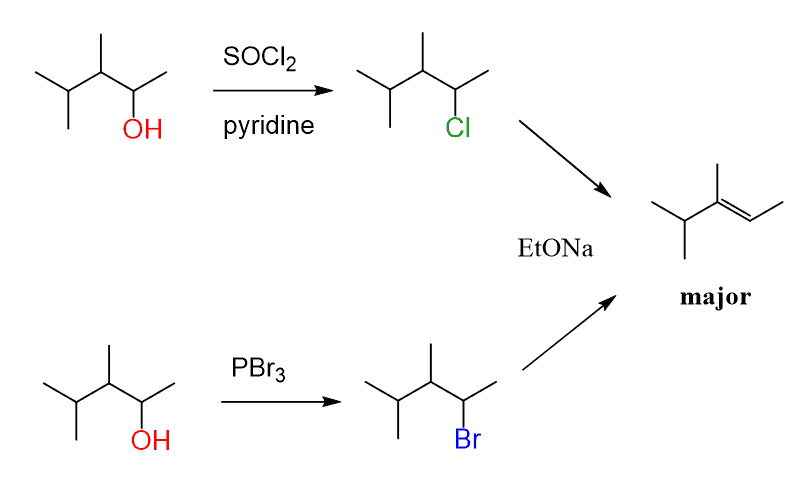
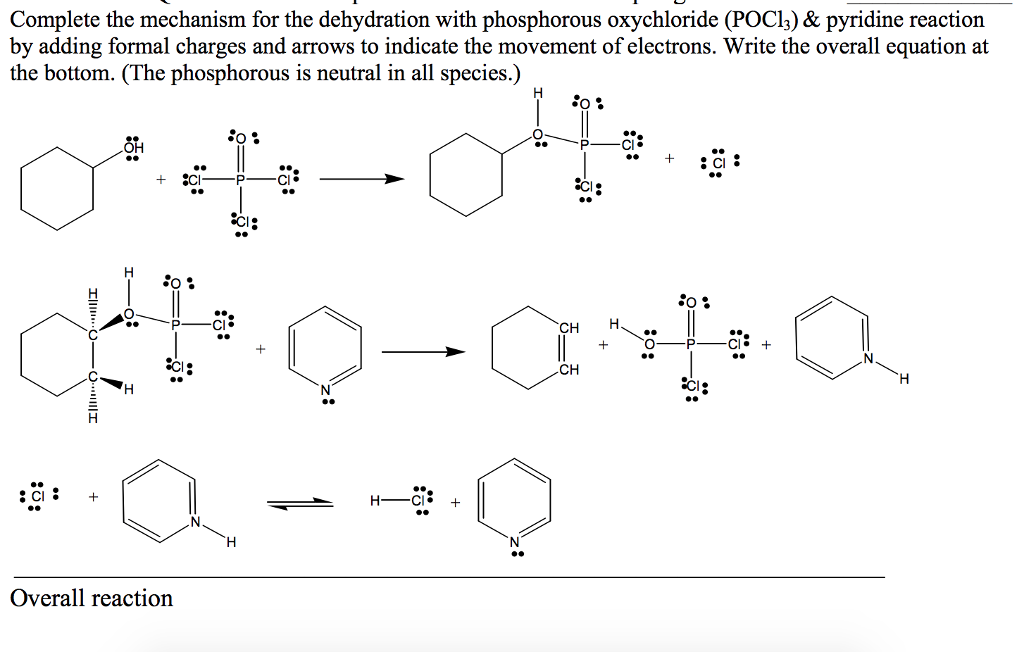
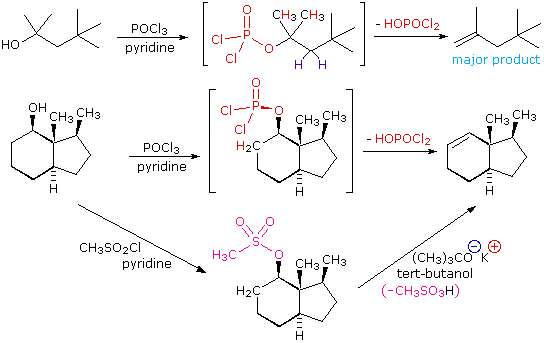
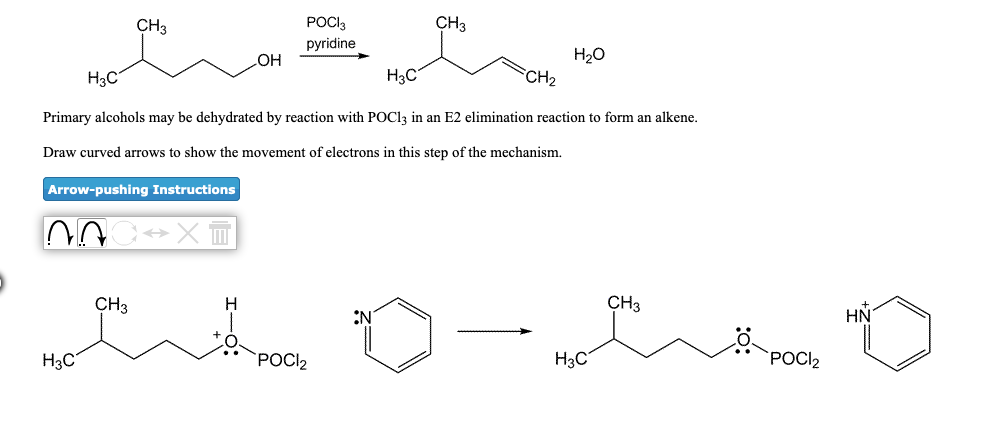
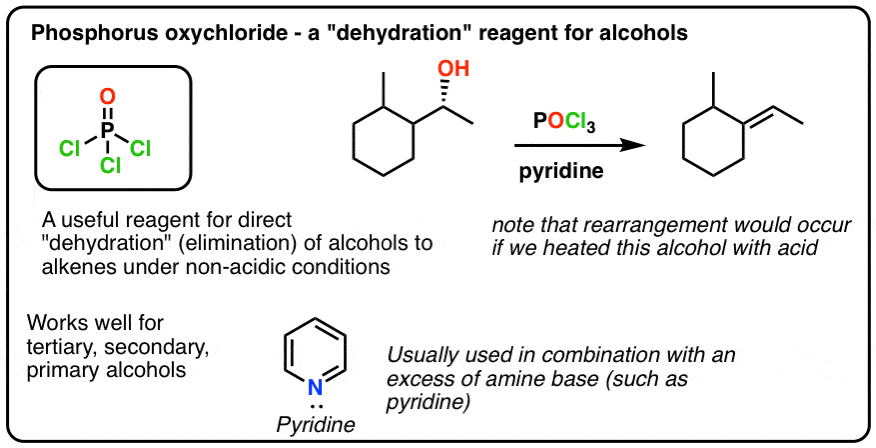
organic chemistry - Is (2R,3S)-3,4-dimethylpentan-2-ol feasible to be dehydrated with POCl3 and pyridine? - Chemistry Stack Exchange

Green Synthesis of Triaryl Phosphates with POCl3 in Water - Huang - 2017 - ChemistrySelect - Wiley Online Library

POCl3‐Promoted Trifluoromethylthiolation‐Based Vicinal Bifunctionalization of Indoles with CF3SO2Na - Sun - 2018 - European Journal of Organic Chemistry - Wiley Online Library

What experimental procedure works best for chlorinating quinazolones using POCl3, POCl3/PCl5, SOCl2/cat. DMF?

POCl3‐Mediated Reaction: A New Entry to Polysubstituted 2‐Pyridones via Self‐condensation of β‐Keto Amides - Gao - 2019 - Journal of Heterocyclic Chemistry - Wiley Online Library

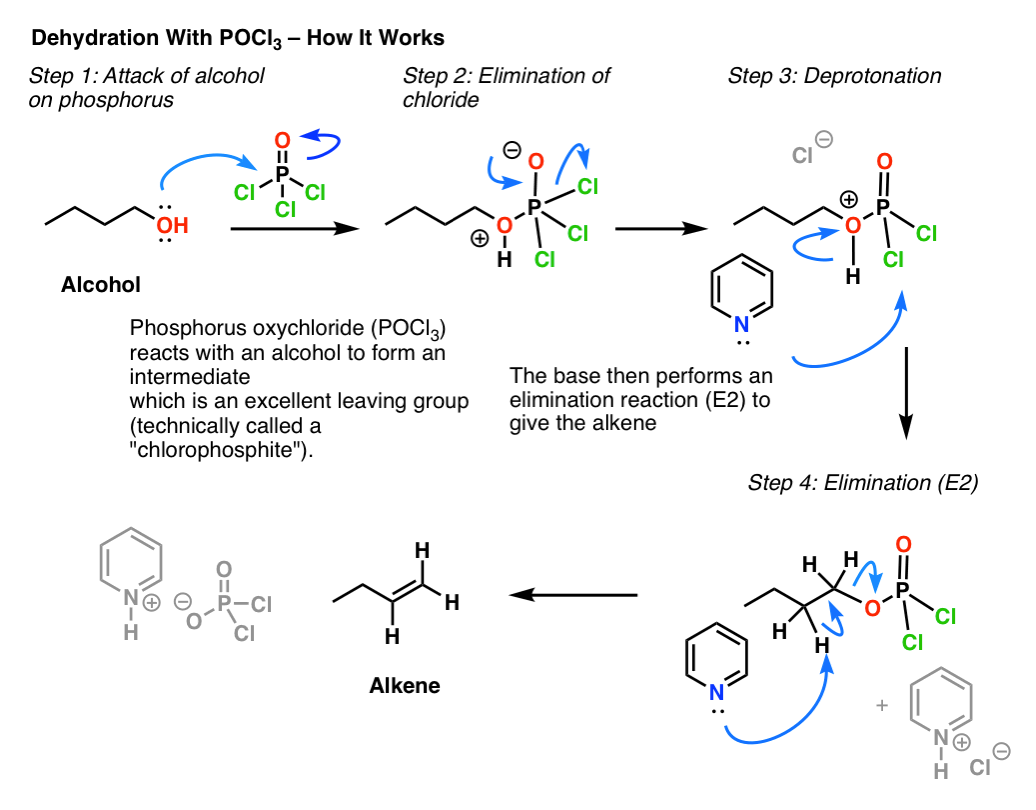
organic chemistry - If SOCl2 reacts with alcohols via SNi, why doesn't POCl3? - Chemistry Stack Exchange
![DMSO–POCl3: a reagent for methylthiolation of imidazo[1,2-a]pyridines and other imidazo-fused heterocycles - ScienceDirect DMSO–POCl3: a reagent for methylthiolation of imidazo[1,2-a]pyridines and other imidazo-fused heterocycles - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0040402013010983-sc1.jpg)
DMSO–POCl3: a reagent for methylthiolation of imidazo[1,2-a]pyridines and other imidazo-fused heterocycles - ScienceDirect

![Alkenes from alcohols [POCl3] - ChemistryScore Alkenes from alcohols [POCl3] - ChemistryScore](https://chemistryscore.com/wp-content/uploads/2019/11/Alkenes-from-alcohols-POCl31-768x336.png)